Purpose of study
The purpose of this study is to validate the use of the EpiSign test in the diagnosis of rare genetic diseases in Canada through collection of real-world evidence. The study will assess the yield, impact and utility of EpiSign in the first stage of diagnosis, as well as a reflex test after a patient’s extensive genetic and genomic testing fails to provide a clear diagnosis.
Who can this study help?
EpiSign can provide diagnostic insight to patients who are suspected of having a syndrome with a defined episignature, or patients with undifferentiated intellectual disability or developmental delay. These patients fall into 2 groups:
- First-visit patients → these patients are genetics naïve or have only received standard microarray and/or fragile X testing
- Reflex patients → these patients have had extensive genetic testing, including large gene panels and whole exome sequencing, but do not have a diagnosis
Sample Requirements
Peripheral blood collected in EDTA (mauve top) vacutainer. Extracted DNA can also be submitted. Please specify concentration and method of extraction, source must be originally from peripheral blood
Study Design
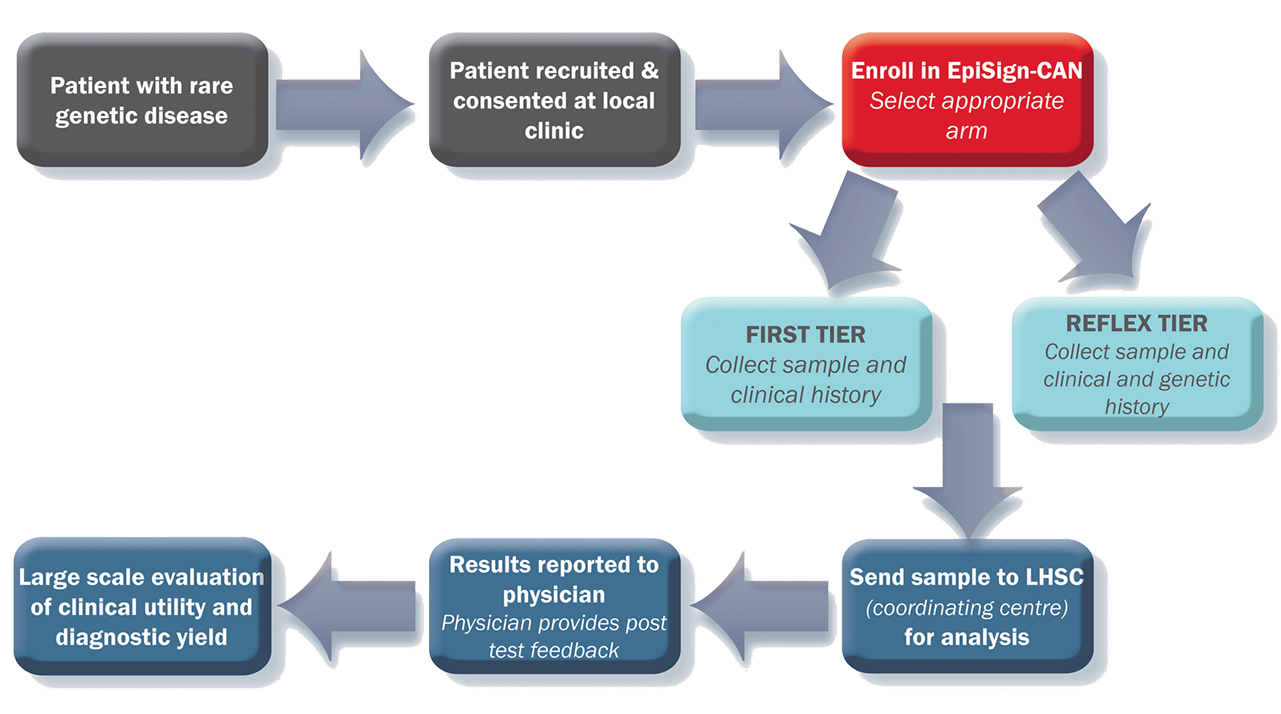
Clinicians and Researchers
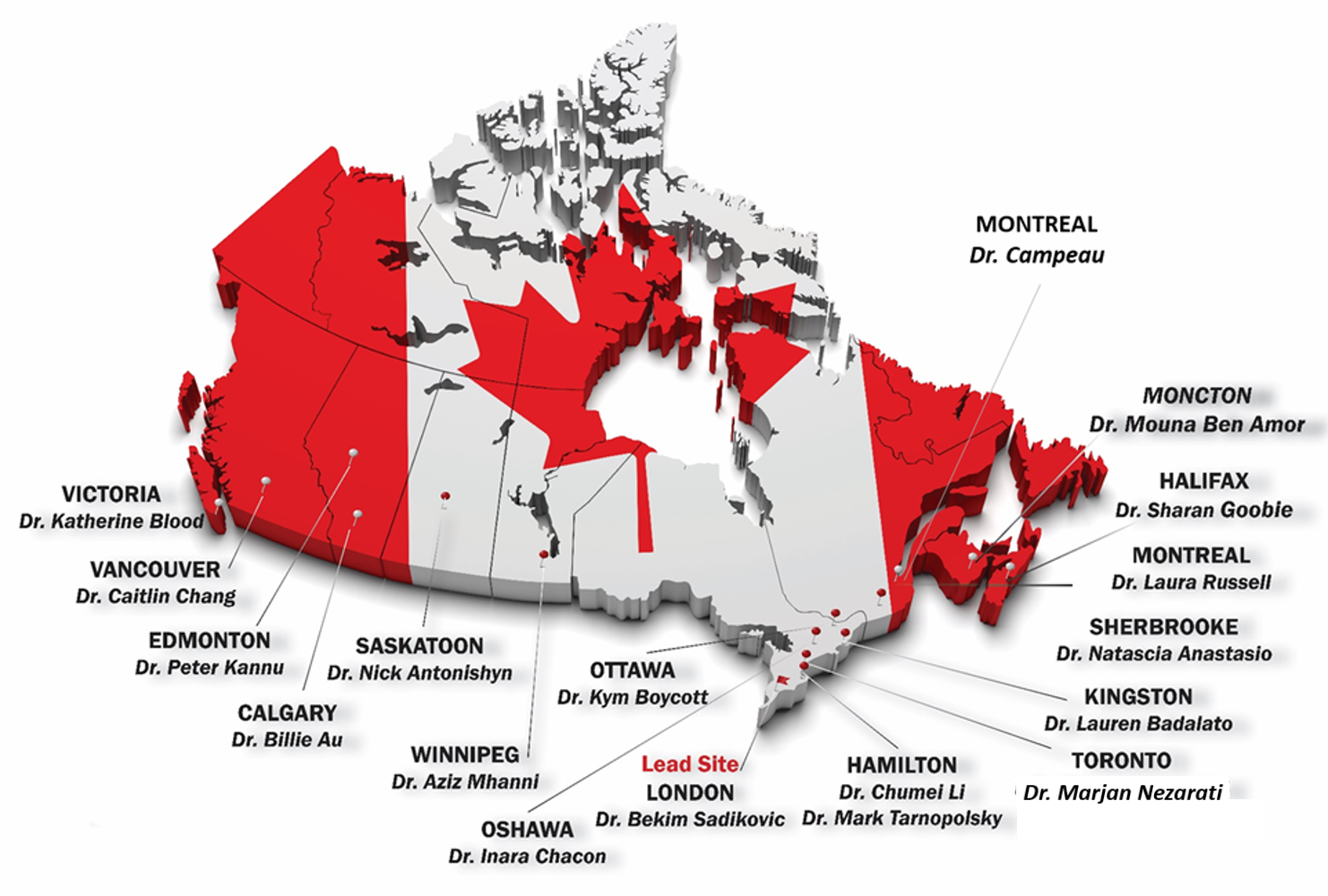
Are you interested in participating in this study? Join the below investigators as a site lead! Please contact the study coordinator, Haley McConkey, with any inquiries.
“Current diagnostic technologies such as microarray and whole exome sequencing are not able to assess non-coding and more complex variants, and cannot provide information on epigenetic changes. This technology provides a new level of analysis beyond the genome.”
– Dr. Bekim Sadikovic



